Rationale
“Microbes are small; their environments are also small.” - T.S. Brock.
Individual microbes (~1 µm) sense and respond to their local microenvironment, which often has a characteristic length scale of ~100 µm. However, microbial communities in the wild are typically studied at human-centric scales (meters or more). This has limited our ability to identify mechanistic drivers of microbial activity, infer meaningful interaction networks, and rationally engineer microbial communities.
Approach
In the ocean, marine detritus (like dead copepods or fish poop) provide nutrient-rich microhabitats where microbial communities can form. Inspired by this example, I have used microbial communities on marine particles as a model system for microscale microbial ecology.

I’ve taken multiple complementary approaches to exploring this topic:

What we’ve learned
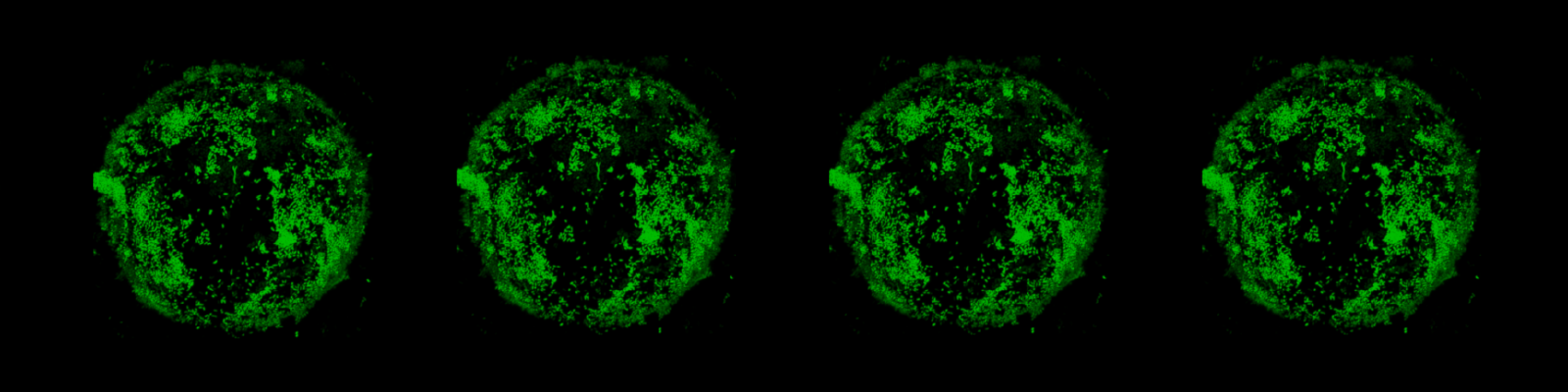
- Marine bacteria form communities on microscale particle microhabitats, including naturally occurring particles (e.g., copepods; Datta*, Almada*, et al., 2018) and synthetic particles in the laboratory (Datta, et al., 2016; Enke*, Datta*, et al., 2018).
- Community assembly occurs via an ecological succession in the laboratory: early stages are dominated by particle-degrading “pioneers”, who provide metabolic byproducts for late-stage colonization (Datta, et al., 2016). We find that this pattern holds for communities formed on a wide range of carbohydrate-rich particles (Enke*, Datta*, et al., 2018).
- Migration on patchy landscapes, like those in the ocean, can promote cooperation in microbial populations. (Datta, et al., 2013).